-
PDF
- Split View
-
Views
-
Cite
Cite
Satoru Suzuki, Nobuyoshi Suzuki, Jun-ichirou Mori, Aki Oshima, Shinichi Usami, Kiyoshi Hashizume, μ-Crystallin as an Intracellular 3,5,3′-Triiodothyronine Holder in Vivo, Molecular Endocrinology, Volume 21, Issue 4, 1 April 2007, Pages 885–894, https://doi.org/10.1210/me.2006-0403
Close - Share Icon Share
Abstract
Previously, we identified reduced nicotinamide adenine dinucleotide phosphate-dependent cytosolic T3 binding protein in rat cytosol. Cytosolic T3-binding protein is identical to μ-crystallin (CRYM). Recently, CRYM mutations were found in patients with nonsyndromic hereditary deafness. Although it has been established that CRYM plays pivotal roles in reserving and transporting T3 into the nuclei in vitro and has a clinical impact on hearing ability, the precise functions of CRYM remain to be elucidated in vivo. To further investigate the in vivo functions of CRYM gene products, we have generated mice with targeted disruption of the CRYM gene, which abrogates the production of CRYM. CRYM knockout loses the reduced nicotinamide adenine dinucleotide phosphate-dependent T3 binding activity in the cytosol of the brain, kidney, heart, and liver. At the euthyroid state, knockout significantly suppresses the serum concentration of T3 and T4 despite normal growth, heart rate, and hearing ability. The disruption of the gene does not alter the expression of TSHβ mRNA in the pituitary gland or glutathione-S-transferase α2 and deiodinase 1 mRNAs in either the liver or kidney. When radiolabeled T3 is injected intravenously, labeled T3 rapidly enters into and then escapes from the tissues in CRYM-knockout mice. These data suggest that because of rapid T3 turnover, disruption of the CRYM gene decreases T3 concentrations in tissues and serum without alteration of peripheral T3 action in vivo.
THE VARIETY OF effects on growth, development, and metabolism attributable to thyroid hormone suggests that this hormone acts through a fundamental mechanism common to many different tissues, yet is capable of multiple functions (1). The pivotal action of T3 is initiated through binding to its nuclear receptors in the target tissues (2). After secretion of the hormone from thyroid gland, T3 travels a long distance to the target. Thyroid hormone moves from outside the plasma membrane into the extranuclear space of the cells through active transporters or by passive diffusion (3). Although many studies demonstrated that cytosolic T3 binding proteins are present in vitro, little is known about the molecular mechanisms of T3 retention in cytoplasm, especially, the physiological roles of cytosolic T3in vivo (4).
In 1986, it was reported that charcoal treatment of rat renal cytosol abolishes the hormone binding activity, whereas the addition of reduced nicotinamide adenine dinucleotide phosphate (NADPH) restores it (5). Two proteins were identified. One was purified from rat kidney (6) and brain (7) and showed a molecular mass of 58 kDa. The other was obtained from rat liver (8), astroglial cells (9), and human kidney (10) and showed a molecular mass of 38 kDa. The latter was identified as μ-crystallin (CRYM), which was initially cloned from kangaroo lens (11, 12). CRYM is a taxon-specific protein that is particularly abundant in kangaroo lens, but not in mouse or other mammals (13). Our previous study demonstrated that CRYM held T3 in cytoplasm, which increased the T3 concentration in both whole cells and the nuclei in CRYM-expressing GH3 cells (14). However, expression of CRYM suppressed transactivity mediated by T3 in the same cell lines.
Recently, it was reported that CRYM is abundant in cochlear and vestibular tissues in the inner ear (15). Immunohistochemical study demonstrated that the protein was preferentially expressed in fibrocyte type 2 where Na-K ATPase is enriched (16). Two patients with nonsyndromic deafness were reported to be associated with point mutations in the CRYM gene (15). In a study assessing NADPH-dependent T3 binding to the two reported mutations (16), one mutation, T314Y, abolished NADPH-dependent T3 binding, whereas the other did not influence the binding activity. The proband with T314Y showed severe hearing loss, whereas the patient with X314T demonstrated moderately impaired hearing. It is suggested that the thyroid hormone binding property may relate to the development of hearing impairment in patients with the CRYM mutations.
To clarify the physiological roles of CRYM in vivo, we generated mice in which the CRYM gene was disrupted. We assessed the phenotypes, especially the heart rate, hearing ability, kinetics of the thyroid hormone, serum concentrations of thyroid hormones, and the mRNA expression of the thyroid hormone response genes.
RESULTS
Production of Mice with Inactivated CRYM
To inactivate the CRYM gene, a recombination cassette containing a NeoR gene driven by mouse phosphoglycerate kinase promoter was introduced in the first and second coding exons (Fig. 1A). The mutated allele was introduced into the CRYM locus by homologous recombination in embryonic stem cells. Heterozygous mice were derived from an inbred C57BL6 background. The chimeric mouse was mated to 129Sv mice and then back-crossed six times into the same strain, diluting the C57BL6 background.
Generation and NADPH-Dependent T3 Binding Activities of CRYM−/− Mice A, Schematic representation of the CRYM gene and targeting. Exon 1 and Exon 2 were disrupted by the substitution for neomycin resistance (NeoR) gene driven by mouse phosphoglycerate kinase promoter and polyA tail. The long fragment of the targeting construct was a 7.3-kb sequence upstream from Exon 1. The short fragment of the targeting construct was 1.2-kb sequence downstream of Exon 2. LA, Long arm, SA, short arm. B, The expressions of the CRYM mRNA and protein. Left panel: Total RNA was extracted from the brain in 3-wk-old wild, CRYM+/−, and CRYM−/− mice. Blotting membrane was hybridized with [32P]dCTP-labeled whole cDNA of the CRYM as a probe. Right panel: Cell lysate was prepared and applied to 10% sodium dodecyl sulfate-polyacrylamide gel. The blotting membrane was incubated with anti-CRYM antibody (L-20) (Santa Cruz Biotechnology). Detection was carried out by measuring the enhanced chemiluminescence using horseradish peroxidase-coupled rabbit antigoat IgG antibody (Amersham Bioscience) (+/+, wild mice; +/−, CRYM+/−; −/−, CRYM−/−). C, Specific T3 binding in the brain, heart, kidney, and liver of CRYM+/+ and CRYM−/− mice. After the tissues were removed from the CRYM+/+ or CRYM−/− mice, crude cytosol fractions were prepared and were incubated with 1% (wt/vol) charcoal for 20 min. After spinning down, the supernatants were incubated with labeled T3 in the presence or absence of NADPH, with or without 10−6m unlabeled T3 for 20 min. After the incubation, dextran-coated charcoal was added, incubated for a few seconds, and spun down to remove charcoal. The supernatant was removed to count the radioactivity. (+/+, CRYM+/+; −/−, CRYM−/−) NADPH− and + denote the incubation without and with 100 μm NADPH, respectively. T3 − and + indicate the incubation without and with unlabeled 10−6m T3, respectively. D, Scatchard analysis of the kidney cytosol obtained from the CRYM+/+ and CRYM−/− mice. The charcoal-treated cytosol was incubated with labeled T3 in the various concentration of unlabeled T3 to perform Scatchard analysis. +/+ Represents the analysis of the CRYM+/+ mice; −/− shows the analysis of the CRYM−/− mice. The representative data were shown. B/F, Bound-free ratio.
Expression of CRYM Gene
Northern blotting demonstrated a single 2.2-kb band in the lane applied brain total RNA from the wild (CRYM+/+) mice (Fig. 1B, left panel). The signal was attenuated in the lane of the CRYM+/− mice. There was no signal detected in the CRYM−/− mice. Western blotting showed a single 38-kDa band in the CRYM+/+ and CRYM+/− mice, but not in the CRYM−/− mice (Fig. 1B, right panel).
NADPH-Dependent T3 Binding Activity
NADPH-dependent T3 binding was assessed in whole brain, heart, kidney, and liver of the CRYM+/+ and CRYM−/− mice. As shown in Fig. 1C, there were no specific bindings to T3 in the charcoal-treated crude cytosol obtained from any of the tissues we studied (black and shaded bars). When we added NADPH to the charcoal-treated extracts, specific binding to T3 was observed in all tissues from the CRYM+/+ mice, as previously demonstrated (white and hatched bars) (17). These binding activities were abolished using extracts from the CRYM−/− mice. Scatchard analyses demonstrated that the association constant (Ka) in the CRYM+/+ mice was approximately 2 × 109/m (Fig. 1D). We could not calculate Ka in the CRYM−/− mice because of the lack of specific binding.
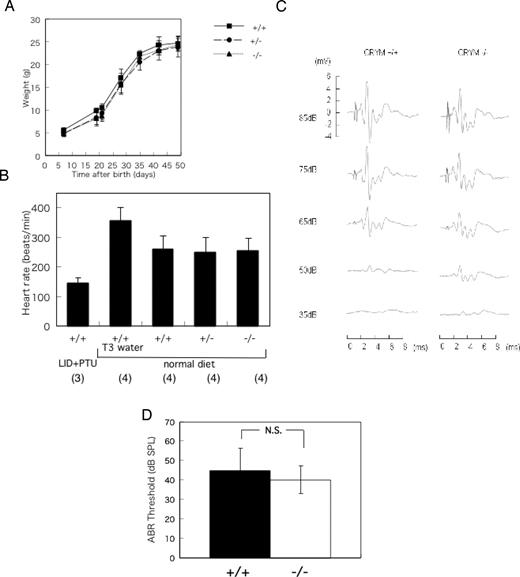
Growth Curve, Heart Beats, and Hearing Ability
The mice were intercrossed to produce homozygous mutants. Among 155 pups born, 34 (22%), 87 (56%), and 34 (22%) were wild-type, heterozygous, and homozygous progenies, respectively. These data show that homozygous disruption of the CRYM gene is not deleterious to embryonic development. The growth rate of heterozygous mice was similar to that of wild-type mice (Fig. 2A). Low and high heart rates were shown in wild-type mice fed a low-iodide diet plus 1% propylthiouracil (PTU) and 1 μg/ml T3 water ad libitum, respectively, as controls. There were no significant differences in the heart rates among CRYM+/+, CRYM+/−, or CRYM−/− mice (Fig. 2B). To assess hearing, auditory-evoked brainstem response (ABR) was measured in CRYM+/+ and CRYM−/− littermates. The responses were apparently normal in CRYM−/− mice (Fig. 2C). The thresholds of CRYM−/− mice were not significantly different from those of CRYM+/+ mice (Fig. 2D).
Phenotype of CRYM−/− Mice A, Growth rate of CRYM+/+, CRYM+/−, and CRYM−/− mice. The data represent mean of three or four male mice ± sd. B, Heart rate of the CRYM+/+, CRYM+/−, and CRYM−/− mice. Male mice (3 wk old) were anesthetized and recorded by the electrocardiogram. The data obtained from CRYM+/+ mice treated with low-iodide diet plus 0.1% PTU (LID+PTU) and 1 mg/ml water ad libitum (T3 water) were shown in the first and second column, respectively. Numbers in parentheses indicate numbers of animals studied. C, Auditory-evoked brainstem response in CRYM+/+ and CRYM−/− mice. The chimeric mouse was mated to 129Sv mice and then back-crossed six times into the same strain, diluting the C57BL6 background. Male mice (6 wk old) were used in this study. The representative data were shown. D, The thresholds of CRYM−/− mice were not significantly different from CRYM+/+ mice. The data were obtained from 6-wk-old male mice. ABR waveforms were recorded in 5- to 10-dB intervals down from a maximum amplitude of 85 dB until no waveform could be visualized. A threshold was defined as the minimal stimulus level that gave a recognizable waveform on a normalized scale. The data represent mean ± sd of six determinations. N.S., Not significant; SPL, sound pressure level; LID, low-iodide diet. P = 0.4178.
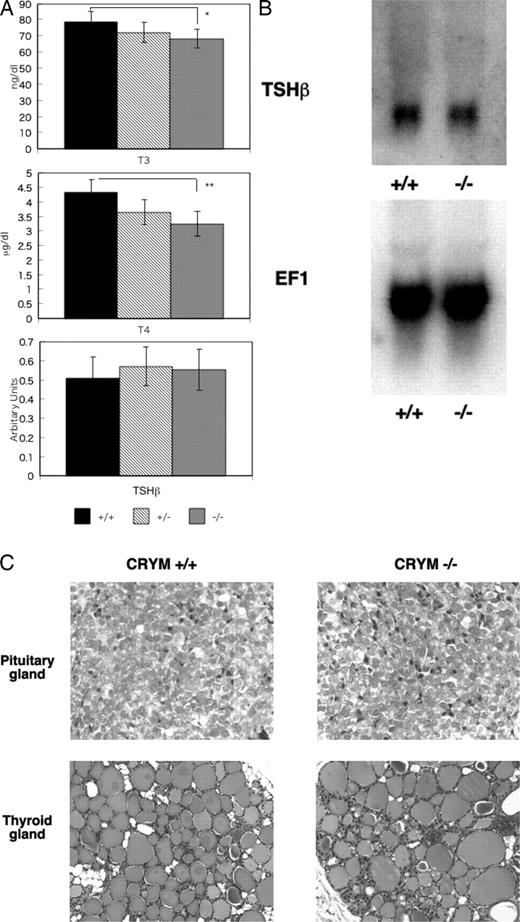
Serum Concentrations of T3, T4 and TSHβ mRNA Expression in the Pituitary Gland
We measured the serum concentrations of T3 and T4 in CRYM+/+, CRYM+/−, and CRYM−/− mice. As shown in Fig. 3A, the concentrations of both T3 and T4 were significantly lower in the CRYM−/− mice than in CRYM+/+ mice. T3 and T4 concentrations were reduced by 13% and 25% in CRYM−/− mice, respectively. In contrast, there was no significant difference in TSHβ mRNA expression between the CRYM+/+ and the CRYM−/− mice. The data from the Northern blotting were consistent with the findings obtained from the quantitated PCR (Fig. 3B). As shown in Fig. 3C, there were no apparent histological differences between CRYM +/+ and CRYM −/− mice in either the pituitary or thyroid gland.
Hormonal Aspects of CRYM−/− Mice A, Serum concentrations of T3 and T4 and the expression of TSHβ mRNA in 3-wk-old male mice. After blood was drawn for the measurement of thyroid hormone, mice were anesthetized with ketamine/xylazyne. The pituitary glands were removed and the total RNA was extracted. The TSHβ and β-actin were quantitated by PCR. All values corrected by the amount of β-actin. Columns indicated mean ± sd from six individual determinations. *, P < 0.05; **, P < 0.01. B, Northern blotting of TSHβ expression in the pituitary glands obtained from CRYM+/+ and CRYM −/− mice. Male mice (6 wk old) were used. Total RNA (10 μg) obtained from six mice was loaded and hybridized with labeled TSHβ or EF1 as an internal control. C, Histology of the pituitary gland and the thyroid gland in CRYM+/+ and CRYM−/− mice. Male mice (8 wk old) were used. Sections were stained with hematoxylin and eosin. The pituitary glands in CRYM +/+ and CRYM−/− mice were shown in left upper panel and right upper panel, respectively. The thyroid glands in CRYM +/+ and CRYM−/− were shown in left lower panel and right lower panel, respectively. There are no apparent differences in both tissues in both genotypes.
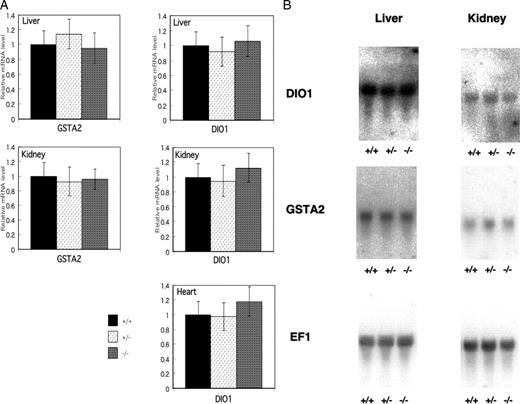
mRNA Levels of T3-Responsive Genes in the Liver, Kidney, and Heart
To evaluate peripheral activity induced by thyroid hormone in knockout mice, we measured the expressions of Gsta2 and Dio1 mRNAs in the liver and kidney and the expression of Dio1 mRNA in the heart. There were no significant differences in Gsta2 or Dio1 mRNA expression in either the liver or kidney among CRYM+/+, CRYM+/−, and CRYM−/− mice (Fig. 4A). In the heart, the expression of Dio1 in CRYM+/+ mice was not different from that in CRYM−/− mice. The expression of Gsta2 was not performed because the expression was too low to evaluate in this system. Northern blotting also demonstrated similar results to the data using the quantitated PCR (Fig. 4B).
The Expressions of Gsta2 and Dio1 mRNA in Liver, Kidney, and Heart of CRYM+/+, CRYM+/−, and CRYM−/− Mice A, Tissues were removed after saline infusion. From each tissues, 50 mg was homogenized and the total RNA was extracted. From each purified total RNA, 20 ng was used for reverse transcription. The mRNAs of Gsta2 and Dio1 were quantitated by sequence detection system. All values were corrected by the amount of 18s rRNA. The columns indicate mean ± sd from four individual determinations. The expression level of Gsta2 in heart was too low to measure in this system. B, Northern blotting of Dio1 and Gsta2 expressions in liver and kidney obtained from CRYM+/+. CRYM+/−, and CRYM−/− mice. Male mice (6 wk old) were used. Total RNA (20 μg) obtained from the tissues in CRYM+/+, CRYM+/−, or CRYM−/− mice was loaded and hybridized with labeled Dio1 or Gsta2. EF1 was used as an internal control.
T3 Kinetics in Wild and Homozygous Mice
To investigate the physiological roles of CRYM in vivo, we injected labeled T3 iv and measured the radioactivity in various tissues 1, 2, 4, and 8 h post injection. In the CRYM+/+ mice, radioactivity in all tissues studied except for the brain gradually increased for 2 h after injection and then slowly decreased until 8 h post injection (Fig. 5A–D). In the brains of CRYM+/+ mice, it took 4 h to reach the peak radioactivity. However, the radioactivities in all tissues peaked 1 h post injection and then sharply declined in the CRYM−/− mice. There were no significant changes in serum radioactivities between the CRYM+/+ and CRYM−/− until 8 h post injection (Fig. 5E).
Radioactivity of the Tissues Removed from Wild (CRYM+/+) and Homozygous Knockout (CRYM−/−) Mice at the Indicated Hours after the Injection of [125I]T3 Mice (6 wk old) were iv injected 91 pmol [125I]T3 via the tail vein. Tissues and blood samples were removed from three or four of each animal at the indicated times after the injection. After the measurement of the tissue weight, radioactivities were counted. The data indicated mean ± sd. (A, brain; B, heart; C, liver; D, kidney; E, blood) *, P < 0.01 vs. study with CRYM+/+ mice.
DISCUSSION
In this study, we succeeded in disrupting the CRYM gene and omitting the expression of the protein in vivo. NADPH-dependent T3 binding activity was eliminated in the tissues we studied, suggesting that the CRYM protein is a unique protein among cytosolic proteins that are capable of binding to T3 in the presence of NADPH. We have reported two proteins of different molecular weights, which possess high affinity to T3 in the presence of NADPH in rat tissues. One was purified from rat kidney and showed a molecular weight of 4.7S, and mass of 58 kDa (6). The other was isolated from rat liver and showed a molecular mass of 38 kDa (8). The molecular weight was 5.1S, because this protein formed a dimer. Currently, we do not have evidence to explain why NADPH-dependent T3 binding completely vanished even though one of the proteins should have survived. CRYM protein may be affected by posttranslational modification, such as glycosylation, myristoylation, or other processes. Such posttranslational modification may alter the molecular weight. As a result, there are two molecular weights of the protein originating from a single protein. Furthermore, the antibody may not recognize the modified protein. These proteins have also been reported (18).
On the initial phenotypical assessment, there were no significant differences in terms of growth rate, heart rate, and hearing ability. Hearing is one of the most important functions controlled by thyroid hormone (19, 20). It is reported that CRYM mutations are associated with nonsyndromic deafness. The disruption of the CRYM gene apparently does not alter the hearing function. From these data, the expression of abnormal CRYM proteins may affect the hearing function of the inner ear in patients with CRYM mutations. We suggest that the mutation that abolishes NADPH-dependent T3 binding causes severe impairment of the hearing as previously reported (16). Taken together, these data indicate that other factors, including thyroid hormone, may also affect hearing loss.
In contrast to normal growth, heart rate, and hearing ability, disruption of the gene decreased the serum concentration of T3 without alteration of the TSHβ concentration in the pituitary gland. T4 concentration was also suppressed by the disruption of the gene. It was reported that the binding affinity of CRYM to T4 is approximately 5–10 times lower than that to T3in vitro (8). These data suggest that CRYM disruption may suppress the T4 concentration directly, or through the feedback loop in TSH-releasing factor-TSH-thyroid axis indirectly in vivo. Because of the small change in the thyroid hormone concentration, we note the possibility that a correspondingly small change exists in the amount of TSHβ mRNA that has not been detected.
Gsta2 and Dio1 are reported to be negatively and positively regulated by T3, respectively (21, 22). Although the concentration of T3 and T4 were low in CRYM−/− mice, the expression level of T3 target genes was normal rather than low in CRYM−/− mice.
A previous study demonstrated that CRYM affected cellular retention of T3 in living cells (14). This observation presupposes that the disruption of the CRYM gene may alter the kinetics of T3in vivo. We counted the radioactivity of the tissues after injection of the labeled T3 iv via the tail vein. Both the influx and efflux of radiolabeled T3 were increased in all of the tissues we studied in these knockout mice. These findings are consistent with the previous data obtained using the CRYM-expressing cells.
Up to the present, four cytosolic thyroid hormone-binding proteins have been reported, i.e. pyruvate kinase subunits (23), aldehyde dehydrogenase (24), glutathione-S-transferase (25), and CRYM. Although the affinity constants of these proteins to T3 are 8–34 times lower than that of CRYM, these proteins may have physiological functions for cytoplasmic T3 retention or transport. MCT8 or other T3 transporters in plasma membrane also contribute to the regulation of intracellular T3 concentration (26, 27).
CRYM proteins may have redundant functions. The protein binds not only T3 but also NADPH (28). The physiological significance of this coupling is not currently known. Because nicotinamide adenine dinucleotide phosphate inhibits T3 binding, oxidative stress may be another factor for control of T3 binding to CRYM in cytoplasm (8).
In conclusion, we disrupted NADPH-dependent T3 binding after the elimination of CRYM expression. Normal growth, heart rate, and hearing were observed. The expressions of TSHβ, Gsta2, and Dio1 were not changed by the disruption, although the serum concentration of thyroid hormone was altered. T3 rapidly entered into and escaped from the tissues. These data suggest that CRYM affects T3 kinetics in vivo and that elimination affects the serum concentrations of T3 and T4 but not the apparent phenotype and peripheral thyroid hormone action in the euthyroid state. The precise molecular mechanism of CRYM action remains to be elucidated.
MATERIALS AND METHODS
Generation of the CRYM Knockout Mouse
The CRYM knockout mouse was generated by in Genious Targeting Laboratory (Stony Brook, NY) on a commercial basis. The CRYM gene was cloned from a 129Sv genomic library (Stratagene, La Jolla, CA) and was used to construct targeting vector containing the neomycin resistance gene flanked by two genomic fragments spanning the CRYM gene promoter, Exon 1 and 2 regions. The long fragment of the targeting construct was a 7.6-kb sequence upstream from Exon 1. The short fragment of the targeting construct was 1.2-kb sequence downstream of Exon 2. The targeted allele contained a deletion of the CRYM gene promoter, Exon 1, and Exon 2. IT2 embryonic stem cells were transfected with the targeting vector and incubated in media containing G418. Surviving colonies were analyzed by PCR to identify homologous recombinants. One correctly targeted embryonic stem cell clone was microinjected into C57BL/6J host blastocysts to generate chimeric mice, which were bred with C57BL/6J mice to obtain heterozygous offspring. Mouse genotyping was performed with genomic DNA isolated from toes using sodium dodecyl sulfate/proteinase K solubilization. The wild-type CRYM allele was identified by PCR using primers a (5′-TGCATCCCTGAAGTGGGGTA-3′) and b (5′-GAATGAGCGCAAACGGAATG-3′), which amplified an 800-base product. The knockout allele was identified by PCR using primers a and c (5′-AGGCAGAGGCCACTTGTGTAG-3′), which amplified a 500-base product-containing fragment of the neomycin-resistant cassette. Heterozygous mice were crossed with the 129Sv strain at least six times before they were interbred to generate homozygous knockout mice. The wild-type mice used in this study as controls were littermates of the knockout mice. Procedures carried out in mice were approved by Shinshu University Institutional Animal Care and Use Committee.
Northern and Western Blotting
In Fig. 1, the brains were obtained from 3-wk-old mice for the Northern and Western analyses. Northern hybridization was performed by the standard procedures using random-primed full length CRYM fragment as a probe (29). In Figs. 3B and 4B, the pituitary gland, liver and kidney were obtained from 6-wk-old male mice. Total RNA (10 μg) obtained from six pituitary glands was electrophoresed and hybridized with 32P-labeled TSHβ or elongation factor 1 (EF1) probe. Total RNA (20 μg) obtained from 50 mg liver or kidney was electrophoresed and hybridized with labeled Dio1, Gsta2, or EF1. A series of the mouse probes were obtained from PCR amplification of the reverse-transcribed poly A RNA extracted from the pituitary gland or liver. The following oligonucleotides were used.
TSHβ sense, 5′-GAACGGAGAGTGGGTCATCACA-3′;
antisense, 5′-AGTAGTTGGTTCTGACAGCCTC-3′;
Dio1 sense, 5′-TGTAGGCAAGGTGCTAATGAC-3′;
antisense, 5′-TGCCGGATGTCCACGTTGTTC-3′;
Gsta2 sense, 5′-GCAGAATGGAGTGCATCAGGT-3′;
antisense, 5′-TCTGCTCTTGAAGGCCTTCAGC-3′;
EF1 sense, 5′-CCATGAAGCTTTGAGTGAAGCTCT-3′;
antisense, 5′-TAGCCTTCTGAGCTTTCTGGGCAG-3′.
Amplified fragment was sequenced and fidelity of the sequences was checked. Western blotting was carried out by using anti-CRYM antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Scatchard Analysis
Scatchard analyses and NADPH-dependent T3 binding assays were performed as previously described (28). Briefly, tissues were homogenized and centrifuged to isolate the crude cytosolic fraction. The fraction was incubated with 1% charcoal to remove T3, NADPH, and other small molecules. After spinning down, the supernatant was incubated with [125I]T3 in the presence or absence of NADPH and/or unlabeled T3 for 20 min on ice. After the brief incubation with charcoal and the following centrifugation, the supernatant and pellet were counted by the γ-counter.
Heart Rate
For the electrocardiogram measurements, mice were anesthetized either with ketamine (100 mg/kg) and xylazine (10 mg/kg) via ip injection as described previously (30). Briefly, mice were positioned prone in a shielded box. The bottom of the shielded box was heated to 37 C using a heating pad to maintain a stable body temperature during the experiment. UAS-108S (Unique Medical, Tokyo, Japan) was used to record bipolar limb leads in standard fashion. For each animal, R-R intervals were measured in a blinded fashion from three consecutive beats in the leads and averaged.
Measurement of Serum T3 and T4
Peripheral blood (400 μl) was corrected from the 6-wk-old male mouse. Serum T3 and T4 were measured by Chemilumi ACS-T3, T4 (Bayer Med, Tokyo, Japan). Six individual mice from each genotype group were used in the study.
ABR
The chimeric mouse was mated to 129Sv mice and then back-crossed six times into the same strain, diluting the C57BL6 background. Male mice (6 wk old) were used in this study. Mice were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg) by ip injection, and the ABR tests were performed with a heating pad to maintain the body temperature in a soundproof room as previously described (31). Click stimuli was performed at synthesized durations and specified amplitudes using a digital signal processing platform (Tucker-Davis Technologies, Alachua, FL), and analyzed with PowerLab systems (ADInstruments, Colorado Springs, CO) as described elsewhere (32). Click stimuli were 0.1-msec clicks, composed of a square pulse of 0.1 msec duration. Stainless steel needle electrodes were placed at the vertex and ventrolateral to the left and right ears for the recording, and a tweeter modified with a coupler was inserted into the external canal to deliver acoustic stimuli. ABR waveforms were recorded in 5- to 10-decibel (dB) intervals down from a maximum amplitude of 85 dB until no waveform could be visualized.
Measurement of TSHβ, Gsta2, and Dio1
The contents of TSHβ, Gsta2, and Dio1 were measured as follows. After iv injection with ice-cold PBS, pituitary glands, livers, and kidneys were removed and later washed with RNA (QIAGEN, Chatsworth, CA). Total RNA was prepared by using RNeasy kit (QIAGEN). After reverse transcription with avian myeloblastosis virus reverse transcriptase, samples were applied with Quantitect SYBR Green PCR kit (QIAGEN) or TaqMan universal PCR Master Mix to ABI PRISM 7300 Sequence detection system according to a manufacturer’s protocol. Mouse TSHβ, β-actin, Dio1, and Gsta2, were measured with the following oligonucleotides.
TSHβ sense, 5′-GCTCGGGTTGTTCAAAGCATGA-3′
antisense, 5′-CCCACAAGCAAGAGCAAAAAGCA-3′
β-actin sense, 5′-TCTCCAGAGGCACCATTGAAATTCT-3′
antisense, 5′-CGCTGGCTCCCACCTTGTCT-3′
Dio1 sense, 5′-CCAGTTCAAGAGACTCGTAGATGAC-3′
antisense, 5′-GCGTGAGCTTCTTCAATGTAAATGA-3′
FAM-TCCACAGCCCATTTC-3′
Gsta2 sense, 5′-AAGACTACCTTGTGGGCAACAG-3′
antisense, 5′-CTGGCATCAAGCTCTTCAACATAGA-3′
FAM-CCTGCTGGAACTTC-3′
The amount of 18s rRNA was measured as described in the manufacturer’s protocol using commercially available probes (Applied Biosystems, Foster City, CA).
Tracer Experiments
Mice were kept under a 12-h light, 12-h dark cycle and provided food and water ad libitum. Three or four CRYM+/+ and three CRYM−/− male littermates were used for each group of treatment. Each mouse was injected iv with 0.2 μCi (91 pmol) of [125I]T3 dissolved into 50 μl of saline. At 1, 2, 4, and 8 h after injection, the mice were killed and tissues were removed, after which the blood samples were drawn. Each tissue and blood sample was weighed and then evaluated for radioactivity with γ-counter.
Statistics
In Figs. 2D and 5, P values were calculated by an ANOVA unpaired t test using Statview 4.1 software (Abacus Concepts, Inc., Berkely, CA). In Figs. 3 and 4, statistical significance was determined by an ANOVA followed by the Bonferroni multiple comparison test. P values ≥ 0.05 were considered not significant.
Acknowledgments
We thank Ms. Izumi Kinoshita for her technical assistance. We are also grateful to Ms. Tomoko Nishizawa for preparation of the tissues.
This work was supported in part by a Grant-in-Aid for Scientific Research no. 17590957 from the Ministry of Education, Science, Sports, and Culture, Japan.
Disclosure Statement: The authors have nothing to disclose.
Abbreviations:
-
ABR,
Auditory-evoked brainstem response;
-
CRYM,
μ-crystallin;
-
dB,
decibel;
-
Dio1,
deiodinase 1;
-
EF1,
elongation factor 1;
-
Gsta2,
glutathione-S-transferase α2;
-
NADPH,
reduced nicotinamide adenine dinucleotide phosphate;
-
PTU,
propylthiouracil.



![Generation and NADPH-Dependent T3 Binding Activities of CRYM−/− Mice A, Schematic representation of the CRYM gene and targeting. Exon 1 and Exon 2 were disrupted by the substitution for neomycin resistance (NeoR) gene driven by mouse phosphoglycerate kinase promoter and polyA tail. The long fragment of the targeting construct was a 7.3-kb sequence upstream from Exon 1. The short fragment of the targeting construct was 1.2-kb sequence downstream of Exon 2. LA, Long arm, SA, short arm. B, The expressions of the CRYM mRNA and protein. Left panel: Total RNA was extracted from the brain in 3-wk-old wild, CRYM+/−, and CRYM−/− mice. Blotting membrane was hybridized with [32P]dCTP-labeled whole cDNA of the CRYM as a probe. Right panel: Cell lysate was prepared and applied to 10% sodium dodecyl sulfate-polyacrylamide gel. The blotting membrane was incubated with anti-CRYM antibody (L-20) (Santa Cruz Biotechnology). Detection was carried out by measuring the enhanced chemiluminescence using horseradish peroxidase-coupled rabbit antigoat IgG antibody (Amersham Bioscience) (+/+, wild mice; +/−, CRYM+/−; −/−, CRYM−/−). C, Specific T3 binding in the brain, heart, kidney, and liver of CRYM+/+ and CRYM−/− mice. After the tissues were removed from the CRYM+/+ or CRYM−/− mice, crude cytosol fractions were prepared and were incubated with 1% (wt/vol) charcoal for 20 min. After spinning down, the supernatants were incubated with labeled T3 in the presence or absence of NADPH, with or without 10−6m unlabeled T3 for 20 min. After the incubation, dextran-coated charcoal was added, incubated for a few seconds, and spun down to remove charcoal. The supernatant was removed to count the radioactivity. (+/+, CRYM+/+; −/−, CRYM−/−) NADPH− and + denote the incubation without and with 100 μm NADPH, respectively. T3 − and + indicate the incubation without and with unlabeled 10−6m T3, respectively. D, Scatchard analysis of the kidney cytosol obtained from the CRYM+/+ and CRYM−/− mice. The charcoal-treated cytosol was incubated with labeled T3 in the various concentration of unlabeled T3 to perform Scatchard analysis. +/+ Represents the analysis of the CRYM+/+ mice; −/− shows the analysis of the CRYM−/− mice. The representative data were shown. B/F, Bound-free ratio.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/mend/21/4/10.1210_me.2006-0403/3/m_zmg0040739970001.jpeg?Expires=1716659090&Signature=ocm8RmQeefJJ4rkWLZDO1sc2oLTNQuJNzAOQQIbAOdbGGLoNqC-M45bkfuTynKMyc668JIFSHAJcX2doxWFiQuDHdAel2cgymbQ3IRghZsu9YvWCyhoU-nYL4fNW3n8fdU0MHwO1DIQeUcrHi8nIGc4IiQpZzs~VULiZ7wM~mkqrRvn7OG62O7-syIH7UcYzQmX~-DINhD8XWuVMz7q7yuBBm1OuJxtdJX7TbOpOuWu3bI4H1GWSFEDVO~WyY-oL29~BV0QalNLimHBtw0Yic5emMO8C34NVAGqpvpkSNTXhuPWlYHMZ1BWQsEwNoVz579wFz6c0q2MPrIaVPI1dOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Radioactivity of the Tissues Removed from Wild (CRYM+/+) and Homozygous Knockout (CRYM−/−) Mice at the Indicated Hours after the Injection of [125I]T3 Mice (6 wk old) were iv injected 91 pmol [125I]T3 via the tail vein. Tissues and blood samples were removed from three or four of each animal at the indicated times after the injection. After the measurement of the tissue weight, radioactivities were counted. The data indicated mean ± sd. (A, brain; B, heart; C, liver; D, kidney; E, blood) *, P < 0.01 vs. study with CRYM+/+ mice.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/mend/21/4/10.1210_me.2006-0403/3/m_zmg0040739970005.jpeg?Expires=1716659090&Signature=oNsNtWJ8-JUJl9eRiiUTwJdRi9KUkh6VeU6zucvV4lGzwQLLAQGZzVn~wNgmtZjzYGfYtWALX7A1qSQoqA-zBVVbRGTHD3HpNOFeNzpGMElWBKgwy90xn~njm6ZBzrxmI0NFcCrY2UymfPP0z5LF8U9~v9dReoGOLvd3a4-qlIat23E~2rpb0jMl2UIGh3Xw26otfNO1O4LQ-0TijuZkgAit~-Q0b74e3rpU17n714bW6VH7Fi6TISzVdORL7KkcpNhyhwikD6yVJoD~8bhAUWgkDkeZfBVCZAu2VpjLipceyCRryqeYPy0PFoH8BokvOTlJ8tXH8epsexZToYz3DQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
